Primary immunodeficiencies (PID) Clinical Update
The content of this document is consistent with ASCIA primary immunodeficiencies (PID) e-training for health professionals.
The main purpose of this document and the ASCIA PID e-training course is to:
- Increase awareness of PID assist health professionals, particularly general practitioners, paediatricians and general physicians.
- Promote early recognition and referral to a clinical immunologist to improve the management and quality of life for patients with PID in Australia and New Zealand.
![]() ASCIA HP Clinical Update PID 2025669.58 KB
ASCIA HP Clinical Update PID 2025669.58 KB
Contents include:
2. Recognition, diagnosis and management of PID
3. Immunoglobulin Replacement Therapy (IRT)
4. Severe Combined Immunodeficiency (SCID)
5. Antibody deficiency disorders
6. Complement disorders including HAE
8. References and further information (See PDF for references)
1. Immunodeficiency overview
How does the immune system work?
The immune system is a collection of tissues (e.g. bone marrow, thymus, lymph nodes and spleen), cells (e.g. lymphocytes, neutrophils and macrophages) and molecules that can be broadly divided into two parts, the innate and adaptive immunity (Marshall et al., 2018). There can be significant overlap between these 2 systems.
Innate immune responses:
- Are present before there is contact with pathogens.
- Are limited in specificity.
- Have no memory or long lived protection.
- Are mediated by cells (e.g. neutrophils, natural killer [NK] cells) and circulating molecules (e.g. complement proteins).
Adaptive immune responses:
- Are slower to initiate.
- Respond specifically to antigens.
- Result in immunological memory.
- Are mediated by cells (T cells, B cells, dendritic cells) and circulating molecules (antibodies).
Effects of age on the immune system
Whilst newborns have maternal antibodies that crossed the placenta during pregnancy, their adaptive immune systems are still developing. Serum immunoglobulin (Ig) levels normally drop as maternal antibodies wane, before recovering as the newborn’s immune system develops (Palmeira et al., 2012).
With advanced age the immune system weakens, particularly the adaptive system, resulting in less efficient responses by T cells and less efficient B cells with less diversity in the immune system (Valiathan et al., 2016). This makes older patients more prone to infection and non-infectious diseases, such as cancer.
What are immunodeficiencies?
Immunodeficiencies are a heterogeneous group of disorders in which there is a defect in the normal function of the immune system (Raje and Dinakar, 2015). Delayed recognition and diagnosis of immunodeficiencies leads to poor health outcomes and potentially premature death.
Infections are the predominant feature of immunodeficiency, particularly infections that may:
- Be unusually persistent, severe, recurrent or resistant to treatment.
- Involve unusual organisms or organisms of usually low virulence.
- Involve body sites not usually prone to infection or multiple organs.
Immunodeficiencies may present at any age and are associated with an increased risk of certain types of autoimmune conditions and malignancies.
There are two types of immunodeficiencies:
- Primary Immunodeficiencies (PID) - There are more than 550 different PID and these are mostly caused by inherited defects of the immune system (Bousfiha et al., 2025). This document focuses on PID.
- Secondary Immunodeficiencies (SID) - These are usually caused as part of another disease or illness (e.g. severe burns, haematological malignancy), as a consequence of certain medications (e.g. chemotherapy) and some infections (e.g. HIV). SIDs are more common than PID and lead to an increased incidence of infection, malignancy or autoimmune disease, similar to PID (Ballow et al., 2022).
Primary immunodeficiencies (PID) overview
PID are inherited defects that affect development and/or function of the immune system and result in increased susceptibility to infection, autoimmunity and malignancy.
More than 550 different immunodeficiency syndromes are now described and whilst individually PID are rare, collectively they make up a significant chronic disease. The prevalence of PID is estimated to be 1 in 1200 but is variable depending on specific disorder (e.g. IgA deficiency occurs in 1 in 500 whilst other PID occur in 1 in 10,000 – 1:1,000,000 people) (Lee and Gray, 2014; McCusker and Warrington, 2011).
More severe forms of PID typically become apparent in early life, such as severe combined immunodeficiency (SCID), with only a short asymptomatic period after birth (Dvorak et al., 2023). Early recognition of severe PID is critical as successful treatment outcomes are dependent on early diagnosis.
PID may present at a later age, with some PID such as common variable immunodeficiency (CVID) more commonly diagnosed in adults (Tam and Routes, 2013).
Despite major advances in molecular characterisation over the last 20 years, many patients remain undiagnosed or are diagnosed late, with adverse effects on morbidity and mortality. Treatment options for PID include prophylactic antibiotics, immunoglobulin replacement therapy (IRT) and in some PID, haematopoietic stem cell transplantation (HSCT), such as bone marrow transplantation (BMT) (Segundo and Condino-Neto, 2021).
PID and the immune system
Although PID vary in many ways, they all result from a defect in one or more elements of immune system regulation or function, as shown in the following table.
|
Classification |
Immune defect |
Examples |
|
Combined immunodeficiencies |
T cells and B cells |
Severe combined immunodeficiencies (SCID) e.g. X-linked SCID, RAG 1 and 2 deficiency |
|
Combined immunodeficiencies with associated or syndromic features |
T cells and B cells with other features |
Ataxia-telangiectasia, DiGeorge syndrome, Wiskott-Aldrich syndrome (WAS), Hyper-IgE syndromes (HIES), DOCK-8, NEMO |
|
Predominantly antibody immunodeficiencies |
B cells or plasma cells |
X-linked agammaglobulinaemia, common variable immunodeficiency (CVID), specific antibody deficiency |
|
Diseases of immune dysregulation |
Susceptibility to haemophagocytosis or defects in regulation of immune system |
Chediak-Higashi syndrome, familial haemophagocytic lymphohistiocytosis (HLH, FLH), X-linked lymphoproliferative syndrome |
|
Congenital defects of phagocytes |
Phagocytes number or function |
Severe congenital neutropaenia, leukocyte adhesion deficiency, chronic granulomatous disease (CGD) |
|
Defects of innate immunity |
Innate immune system |
IRAK-4 and MyD88 deficiencies, WHIM syndrome, TLR3 and UNC93B1 deficiencies, chronic mucocutaneous candidiasis |
|
Autoinflammatory disorders |
Cytokine overproduction |
Familial Mediterranean fever, Hyper-IgD syndrome, Muckle-Wells syndrome, familial cold autoinflammatory syndrome |
|
Complement immunodeficiencies |
Complement components |
Deficiencies of components of the classic or alternate complement pathway (e.g. C5 or C6 deficiency), C1 inhibitor deficiency / hereditary angioedema (HAE) |
|
Phenocopies of PID |
Arise from acquired mechanisms |
(a)Phenocopies associated with somatic mutations e.g. autoimmune lymphoproliferative syndrome (ALPS-SFAG) (b) Phenocopies associated with autoantibodies e.g. chronic mucocutaneous candidiasis due to IL-17 autoantibody, adult-onset immunodeficiency due to INF-gamma autoantibody, pulmonary alveolar proteinosis due to Granulocyte-macrophage colony-stimulating factor (GM-CSF) autoantibody |
Raje and Dinakar (2016)
2. Recognition, diagnosis and management of PID
There are seven early warning signs of PID (McCusker et al., 2018):
- An unusually large number of infections requiring treatment including:
- Frequent middle ear infections (>8 per year).
- More than one serious sinus infection per year.
- More than one pneumonia infection per year.
- Chronic suppurative sinus or lung disease in adults.
- Infections caused by unusual or opportunistic types of organisms including:
- Organisms of low pathogenicity.
- Infections in unusual places including:
- Perianal abscesses
- Organ abscesses
- Infections that do not respond to treatment as normally expected, including:
- Infections refractory to antibiotic treatment.
- Rapid recurrence of infections after ceasing antibiotic treatment.
- Persistent unexplained oral thrush after one year of age.
- Severe infections (e.g. meningitis, osteomyelitis, pneumonia) requiring IV antibiotics.
- Middle ear infections asssociated with a persistent purulent discharge.
- Recurrent serious sinus infections.
- Persistent production of purulent sputum.
- A child that does not grow or put on weight as expected, or weight loss in adults.
- A family history of immunodeficiency or abnormal infections, particularly if there is:
- Shared kinship of parents.
- A family history of fatal infective complications or unexplained infant deaths.
- Any other unusual symptoms related to infections, including:
- Persistent diarrhoea.
- Unusual infection related sequelae or syndromes.
Other features of PID (these mainly occur in adult patients with CVID) include :
- Autoimmune cytopenias.
- Granuloma formation at any site (often misdiagnosed as sarcoidosis).
- Chronic enteropathy unresponsive to gluten withdrawal from diet.
- Unexplained hepatomegaly especially in adults.
- Unexplained splenomegaly in children or adults.
- Lymphoid interstitial pneumonitis.
Delay in diagnosis is common
Severe PID may be recognised in early childhood. However, many PID are undiagnosed into adolescence or adulthood. According to a survey by the Immue Deficiencies Foundation (IDF) in 2007 amongst 1,300 people with PID, the average time from symptom onset to diagnosis is 12.4 years (Condino-Neto and Espinosa-Rosales, 2018).
The role of GPs, paediatricians and physicians in identifiying and managing patients with PID
GPs, paediatricians and adult medicine physicians, particularly respiratory medicine physicians and gastroenterologists, have an important role in identifying and managing patients with PID, including (O’Keefe et al., 2016):
- Recognition of early symptoms and signs of PID.
- Appropriate investigation and interpretation of test results.
- Appropriate and timely referral to a clinical immunologist.
- Appropriate follow up care, in conjunction with a clinical immunologist, particularly assessment of growth and development in children.
- Management of general health issues of patients with PID.
Expected frequency of infections in childhood
The burden of infectious disease is high in children, even in individuals with healthy immune systems. In Australia normal healthy toddlers can have up to 4-8 minor infections each year. The number of infections can be increased up to 12 if the child attends childcare, has older siblings or has parents who smoke.
PID should be considered in patients with recurrent infections (more than expected for age), severe infections or infection with unusual or opportunistic organisms.
Frequency of infections in general childhood population:
|
Age (yrs) |
Infections/yr |
|
0-2 |
6 |
|
3-4 |
5 |
|
5-9 |
4 |
|
10-14 |
3 |
Gray et al. (2010)
Testing for PID
Initial evaluation always starts with a thorough clinical history and physical examination.
Prediction of specific PID on the basis of a particular organism is difficult. However, identification of certain types of microbial organisms may help provide clues as to the underlying defect in the immune system, as shown in the following table.
Infections associated with PID
|
PID |
Infections |
Organisms |
|
Cellular (combined T and B cell) immunodeficiencies |
Systemic viral infections, intracellular bacteria, fungal infections |
Bacteria: Pyogenic bacteria |
|
Viruses: All |
||
|
Mycobacteria: Non-TB including Bacillus Calmette-Guerin (BCG) |
||
|
Fungi: Candida, Aspergillus |
||
|
Protozoa: Pneumocystis jiroveci pneumonia (PJP), cryptosporidium |
||
|
Antibody (B cell) deficiencies |
Upper and lower respiratory tract infections, GI tract infections |
Bacteria: Streptococcus pneumonia (pneumococcus), Haemophilis influenzae, Moraxella catarrhalis, Campylobacter |
|
Viruses: Enteroviruses |
||
|
Protozoa: Giardia |
||
|
Phagocytic immune defects |
Respiratory tract, cellulitis, skin abscesses, liver abscesses |
Bacteria: Staphylococcus aureus, Pseudomonas aeruginosa |
|
Mycobacteria: Non-TB including BCG |
||
|
Fungi: Candida, Aspergillus |
||
|
Complement immunodeficiencies |
Meningitis, systemic bacterial infections |
Bacteria: Neisseria, Streptococci |
Oliveira and Fleisher (2010)
Antibody (B cell) deficiencies
Antibody deficiencies are the most common type of immunodeficiency and are associated with decreased ability to produce protective antibodies against pathogens. They usually present from three months after birth, once maternal antibodies transferred across the placenta have gone (Ozdemir, 2021).
Patients with antibody or humoral deficiencies generally have more frequent and/or prolonged infections compared to healthy individuals. Typically these patients suffer recurrent or severe bacterial infections of ears, sinuses and lungs, particularly with encapsulated organisms (e.g.pneumococcus, meningococcus, haemophilus) (Oliveira and Fleisher, 2010).
Evaluation of antibody (B cell) deficiencies
- Antibody function can be tested both quantitatively and qualitatively.
- Quantitative serum immunoglobulin (Ig) tests are used to detect abnormal levels of the 3 major classes of Ig – IgG, IgA and IgM (Loh et al., 2013).
- A low level of IgG is termed ‘hypogammaglobulinaemia’ and its presence should be confirmed on repeat testing. Any underlying secondary causes of hypogammaglobulinaemia should be considered before referral to a clinical immunologist (Anderson et al., 2022).
- Cellular immune testing for B cells should be performed in consultation with a clinical immunologist.
- Assessment of specific antibody responses (e.g. tetanus and pneumococcus) may be required, usually in consultation with a clinical immunologist, in less severe forms of hypogammaglobulinaemia or when the clinical history is suggestive of an antibody deficiency disorder (Thong et al., 2023). Specific antibodies need to be correlated with vaccine history and age of patient.
|
Patients with abnormal results or patients with normal results who are of ongoing clinical concern should be referred to a clinical immunologist. |
Cellular (combined T and B cell) immunodeficiencies
Cellular immunodeficiencies are much less common than antibody immune defects and are often present with defects of B cells and other immune cells (Buckley, 2002). Therefore they are sometimes called combined immunodeficiencies. Primary T cell deficiencies present early in life, with failure to thrive, opportunistic infections, candida and chronic diarrhoea.
|
If a cellular immunodeficiency is suspected, live vaccines need to be withheld and patient should be referred to a clinical immunologist for an urgent consultation. |
Evaluation of cellular (combined T and B cell) immunodeficiencies
Tier 1 (GPs, paediatricians)
- Full blood count
- Particularly in children, transient lymphopenia is common. Persistent or profound lymphopenia should be followed up.
- T cells comprise two thirds of lymphocytes in blood, therefore decreased T cells are typically associated with lymphopenia on a full blood count but normal T cells does not rule out cellular immunodeficiency.
- Quantitative serum Ig tests are used to assess humoral immunity.
Tier 2 (Clinical immunologists and/or paediatricians)
- Flow cytometry for T cells, B cells and NK cells.
- HIV testing should be performed to exclude HIV.
- Additional testing may be indicated.
Lee and Gray (2014)
|
Severe T cell immunodeficiencies such as SCID are medical emergencies and patients need to be urgently referred to a clinical immunologist for continuation of diagnosis and treatment. (Firatoglu et al., 2025) |
Phagocyte immune defects
Phagocyte abnormalities are due to defects in neutrophils or monocytes/macrophages and are commonly present with recurrent pyogenic or fungal skin infections and/or abscesses (Mickey et al., 2024).
Evaluation of phagocyte immune defects
Tier 1 (GPs, paediatricians)
- Full blood count with blood film can assess for neutropenia although multiple time points are needed to exclude conditions with fluctuating neutrophil counts.
- Blood films may show disorders associated with abnormal granules inside phagocytes.
Tier 2 (clinical immunologists)
- If neutrophil numbers are normal, nitroblue tetrazolium (NBT) and/or dihydrorhodamine (DHR) tests may be used to assess for function.
- Additional testing may be indicated.
Mickey et al. (2024)
Complement immunodeficiencies
Complement immunodeficiencies may be due to deficiencies in any on the components of the 3 complement pathways (McMurray et al., 2024).. Clinical indications include bacterial infections (especially Niesseria), autoimmunity and recurrent angioedema.
Evaluation of complement immunodeficiencies
Initial screening should include C3, C4, AH50 and CH50. In patients with recurrent angioedema C4 and C1 esterase levels should be tested (Bonilla et al., 2015). Additional testing including individual components may be indicated in consultation with a clinical immunologist.
When should patients be referred to a clinical immunologist?
Patients should be referred to a clinical immunologist when (O’Keefe et al., 2016):
- They have early warning signs of PID
- Results of initial screening suggests PID
- Results of initial screening are confusing and diagnosis is unclear
- Results do not confirm PID but there remains a high clinical suspicion for PID
Additional testing includes specialised molecular and genetic tests, that may be needed to confirm a diagnosis.
General care of PID patients
Management of patients with PID requires a multidisciplinary team.
Issues include:
- Monitoring growth and ensuring adequate nutrition in children (Karhan et al., 2022).
- Optimising exercise, sleep and stress management.
- Ensuring good general hygiene (e.g. hand washing, dental hygiene)
- Providing information about patient support organisations:
- AusPIPS - auspips.org.au
- HAE (Hereditary Angioedema) Australasia – haeaustralasia.org.au
- Immune Deficiencies Foundation Australia (IDFA) – idfa.org.au
- Immune Deficiencies Foundation New Zealand (IDFNZ) – idfnz.org.au
Specific therapies for PID
The following therapies need to be individualised according to the type of PID (Segundo and Condino-Neto, 2021):
- Prophylactic antibiotics, antifungals, antivirals.
- Immunoglobulin replacement therapy (IRT).
- Immune modulatory drugs.
- Gamma interferon.
- Haemotopoetic stem cell transplant (HSCT).
- Bone marrow transplantation (BMT).
- Gene therapy.
- Others – granulocyte-colony stimulating factor (G-CSF), polyethylene glycol-conjugated adenosine deaminase (PEG-ADA).
Infections in PID patients
Infections should be investigated and treated aggressively. It is important to (Refaat et al., 2024):
- Notify microbiology laboratory to consider atypical or opportunistic infections.
- Treat patient with broad spectrum antibiotics until organism is identified.
- Consider fungal, mycobacterial, viral and protozoan therapy, according to specific condition.
Serology is not useful if patient is unable to make antibodies or is on immunoglobulin replacement therapy (IRT).
Curative therapies
Haemotopoetic stem cell transplant (HSCT)
Potential sources of stem cells include bone marrow, peripheral blood or umbilical cord blood (Castagnoli et al., 2019).
Gene therapy
This therapy is still experimental and is therefore not routinely available. It involves the use of viral organisms to replace the missing genes and correct the abnormality (Houghton and Booth, 2021).
PID and vaccinations
If a PID is suspected or confirmed, live attenuated vaccines should not be routinely administered as they may cause disease in immunodeficient patients. Issues regarding immunisation of these patients should be discussed with a clinical immunologist (Sobh and Bonilla, 2016).
Potential live vaccines include (Shearer et al., 2014):
- MMR
- Varicella
- BCG (given to at risk populations).
- Rotavirus
- Yellow fever.
Meningococcal, pneumococcal and Haemophilus Influenza vaccines are recommended for patients with complement immunodeficiencies.
Current immunisation guidelines (ATAGI, 2022): https://immunisationhandbook.health.gov.au/
Vaccination information for patients on immunoglobulin replacement therapy (IRT): https://immunisationhandbook.health.gov.au/contents/vaccination-for-special-risk-groups/vaccination-for-people-who-have-recently-received-normal-human-immunoglobulin-and-other-blood-products
3. Immunoglobulin replacement therapy in PID
Immunoglobulin replacement therapy (IRT)
Replacement of serum immunoglobulin with human gamma globulin fraction is the standard treatment for most patients with hypogammaglobulinaemia and some with specific antibody deficiencies (Sriaroon and Ballow, 2015). The aim is to replace immunoglobulin to maintain normal IgG levels. The dose used is individualised for each patient.
IRT may be given as intravenous immunoglobulin (IVIg) or subcutaneous immunoglobulin (SCIg) and pharmacokinetics differ according to administration route (Mallick et al., 2025). There are multiple brands that may change from time to time and rates of administration vary for different products.
Immunoglobulin products
Immunoglobulin products are made from pooled plasma from many healthy human donors (Fernández-Cruz et al., 2009). Plasma donors and plasma pools are screened for hepatitis B, hepatitis C and HIV. Additional viral inactivation steps such as heat treatment, enzyme treatment, detergent treatment and nanofiltration are performed.
Despite differences in manufacturing, all IgG products contain 97-98% IgG specific antibodies against broad spectrum of bacterial and viral pathogens, with traces of IgM and IgA.
Long term benefits of immunoglobulin replacement therapy
Both intravenous immunoglobulin (IVIg) and subcutaneous immunoglobulin (SCIg) (Jolles et al., 2005):
- Are effective at reducing infections and hospitalisations.
- Preserve organ function and reduce long term damage from recurrent infections.
- Are associated with significant benefits to patient quality of life.
- Improve the lifespan of patients.
Intravenous Immunoglobulin (IVIg) replacement therapy
Intravenous immunoglobulin (IVIg) replacement therapy (Li and Mahmood, 2024):
- Is usually administered three to four weekly in hospital.
- Leads to a high peak of IgG after infusion.
- Levels decrease rapidly over few days then slowly decrease over next few weeks.
The majority of side effects of IVIg are mild and self-limiting and include headache, fever, chills, nausea, fatigue or flu-like illness (Guo et al., 2018). The frequency of side effects of IVIg may be linked to the rate of infusion.
Serious adverse events are rare but include anaphylaxis, aseptic meningitis, renal impairment and thrombosis.
Subcutaneous Immunoglobulin (SCIg) replacement therapy
Subcutaneous immunoglobulin (SCIg) requires frequent administration (one to three times per week) by patients or carers at home. SCIg involves slow diffusion of IgG from subcutaneous tissue and the small and more frequent doses are associated with more consistent IgG levels (Jolles et al., 2015). Whilst systemic reactions to SCIg are much less common than with IVIg, local injection site reactions (e.g. redness, itching, swelling, discomfort) are common but improve with time (Cinetto et al., 2021).
SCIg injections may be administered at multiple possible sites according to patient preference. Usually the lower abdomen will be used. However, the outer edge of the thigh or back of the upper arm can also be used for SCIg infusions (Gungoren et al., 2024). The shaded areas below can be used for insertion of the needle.
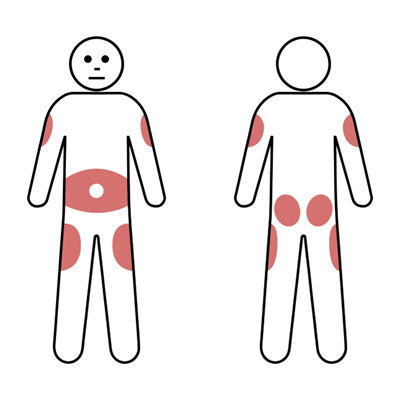
IVIg versus SCIg replacement therapy
The choice of route (IVIg or SCIg) is dependent on multiple factors. Treatment is tailored to patient preference, medical conditions and lifestyle. The preferred route may vary at different times during a patient’s life.
Comparison of Pros and Cons of IVIg and SCIg therapy Source: Adapted from APIIEG
|
|
Pros |
Cons |
|
IVIg |
|
|
|
SCIg |
|
|
4. Severe combined immunodeficiency (SCID)
Severe combined immunodeficiency (SCID) is a rare inherited condition, with six to eight new patients diagnosed in Australia and New Zealand each year (Richards et al., 2020).
SCID is (Heimall et al., 2017):
- The most severe form of PID and has a high mortality, especially if diagnosis is delayed.
- Usually fatal within the first 2 years without treatment.
- Caused by genetic defects affecting the number or function of T cells.
- Characterised by absence or dysfunction of T lymphocytes affecting both cellular and antibody mediated immunity. Depending on the type of SCID, B cells and NK cells may be present or absent.
- A syndrome caused by mutations in one of at least 10 genes affecting T cell development.
The only cure routinely available for SCID is Haemotopoetic stem cell transplant (HSCT). Gene therapy trials are in progress (Wadbudhe et al., 2023).
Approximately half of all SCID cases are X linked due to mutations in the common gamma chain (Tuovinen et al., 2020). Other forms of SCID include deficiency of the enzyme adenosine deaminase (ADA) and a variety of other genetic defects (Kuo et al., 2020). Atypical SCID patients with partial function of genes typically associated with classical SCID may occur and may present at later ages (Dvorak et al., 2023).
Infants affected by SCID generally appear well at birth but are usually symptomatic within the first few months of life. Symptoms include (Aranda et al., 2024):
- Recurrent severe infections, which may be life-threatening.
- Chronic diarrhoea.
- Poor growth and failure to thrive.
- Recurrent or persistent oral thrush, viral respiratory and gastrointestinal infections.
- Opportunistic infections, particularly PJP or disseminated BCG.
- Extensive skin rashes such as erythroderma or eczema.
- Absent lymphoid tissue.
Early diagnosis of SCID is important
Early diagnosis of SCID is critical as early treatment is associated with improved outcomes. In some countries (including Australia and New Zealand) early diagnosis is facilitated by newborn screening programmes (Chan et al., 2011; Heather et al., 2022; Chong-Neto et al., 2024).
Tests for diagnosis of SCID include (Dvorak et al., 2023):
- Full blood count to assess for lymphopenia (normal result does not rule out SCID).
- Flow cytometry for enumeration of T cells, B cells and NK cells.
- Quantitative serum Ig tests are used to assess antibody mediated immunity.
- Chest X-ray may show absence of thymic shadow.
- HIV testing should be performed to exclude HIV.
- Additional testing may be indicated including naïve and memory T cells, in vitro T cell proliferation to mitogens and specialised cellular and molecular tests, which should only be requested after discussion with a clinical immunologist.
|
SCID is a medical emergency and patients need to be urgently referred to a clinical immunologist in a specialist centre for assessment and treatment. |
Management of SCID
Management of patients with SCID includes (Kohn et al., 2019):
- Aggressive search and treatment of infections.
- Protective isolation, usually in hospital.
- Immunoglobulin replacement therapy (IRT).
- Prophylaxis regime designed to prevent opportunistic bacterial, viral and fungal infection (Fischer, 2000):
- Cotrimoxazole for PJP.
- Fluconazole for fungal infections.
- Acyclovir for viral infections.
Other issues (Kohn et al., 2019):
- Live vaccines should not be given to the patient or their family.
- Breastfeeding should be withheld until mother’s Cytomegolovirus (CMV) status is known.
- Additional nutritional support may be required.
- All blood products must be irradiated, leukodepleted (GVHD) and CMV negative.
- For ADA deficiency enzyme replacement with pegylated-ADA is possible although this is not curative.
Curative therapy
- Haematopoetic stem cell transplant (HSCT) is currently the treatment of choice and a successful procedure is generally curative (Castagnoli et al., 2019).
- Gene therapy is available in a few specialised centres as part of clinical trials (Houghton and Booth, 2021).
Haematopoetic stem cell transplant (HSCT)
HSCT provides a new source of normal lymphocytes, especially T cells. Stem cell sources used include bone marrow, peripheral blood and umbilical cord blood.
The success of HSCT varies according to the (Castagnoli et al., 2019):
- Type of SCID.
- Number of infections, especially around the time of the transplant.
- Type of treatment the stem cells has to receive to reduce the risk of being rejected, such as removal of the T cells (or 'T cell depletion').
- Need for conditioning, whereby the bone marrow of the patient with SCID is suppressed prior to transplantation, to improve the ability of the new marrow to grow properly (or 'take').
- Source of the stem cells - transplantation of bone marrow cells from a family member with identical 'tissue typing' of human leukocyte antigens (HLA) gives best results.
Some patients may not achieve B cell engraftment and may require lifelong IRT (Zimmerman and Shenoy, 2020).
5. Antibody deficiency disorders
Common variable immunodeficiency (CVID)
- Most frequent clinically symptomatic PID.
- Mixed group of disorders with similar presentation.
- Relative prevalence between 1:10 000 and 1:50 000.
- Affects both males and females equally.
- Generally be sporadic with <10% of cases familial.
- Some genes have been identified but most cases remain unidentified.
Dahl et al. (2023)
CVID characteristics
- Clinical features variable and infections, autoimmunity, granulomatous disease and lymphadenopathy.
- Have low immunoglobulins of at least 2 isotypes (IgG and either IgA or IgM).
- Associated with poor specific antibody responses:
- Poor antibody response to vaccines and/or absent isohaemagluttinins.
- Disease onset may occur at any age:
- Most patients present with symptoms between ages of 20-40 years.
- ~20% diagnosed in childhood.
- Delayed diagnosis common: four to nine years between symptom onset and diagnosis is usual.
- Usually normal B cell numbers but may be low.
- Variable T cell abnormalities in some.
Cunningham-Rundles (2012)
CVID diagnosis
Diagnosis of CVID can be given to a patient if the patient is over 4 years of age and has all of (Lee et al., 2021):
- Consistent clinical features (infections, autoimmunity, granulomatous disease and lymphadenopathy).
- Marked decrease of IgG and marked decrease of IgA and/or IgM levels.
- Poor response to vaccines (and/or absent isohaemaglutinins).
- Secondary causes of hypogammaglobulinaemia have been excluded.
Clinical features of CVID
- Infections – most common (90%) with sinopulmonary, ear and gastrointestinal infections.
- Gastroenterological (up to 50%) – chronic diarrhoea, malabsorption.
- Lymphophadenopathy or splenomegaly (50%).
- Autoimmunity (30%).
- Granulomas (10-30%) – lungs, liver, other.
- Splenomegaly (20%).
- Malignancy – increased incidence of lymphoma and stomach cancers.
Bonilla et al. (2016)
CVID treatment options
- Regular and sufficient substitution with immunoglobulins: 90% of CVID patients require either IVIg or SCIg.
- Dose of immunoglobulin to maintain normal serum IgG levels and keep patient infection free.
- Prophylactic antibiotics in patients with chronic suppurative lung disease or frequent infections.
- Targeted antibiotic treatment of (breakthrough) infection.
- Antibiotic treatment may need to be higher dose and longer duration than normal healthy individuals.
- Appropriate treatment of complications (e.g. bronchiectasis, sinusitis, enteropathy, autoimmunity).
- Surveillance for autoimmune disease including cytopaenias, granulomatous disease and adenopathy.
- Surveillance for malignancy, particularly including lymphoproliferative disease and gastric cancer.
Salzer et al. (2012)
Differential diagnosis of hypogammoglobulinaemia
|
Genetic Disorders |
Drug Induced |
Infectious Diseases |
Malignancy |
Systemic Disorders |
|
Ataxia Telangiectasia |
Antimalarial agents |
HIV |
Chronic Lymphocytic Leukemia |
Immunodeficiency caused by hypercatabolism of immunoglobulin |
|
Autosomal forms of SCID |
Captopril |
Congenital Rubella |
Immunodeficiency with Thymoma |
Immunodeficiency caused by excessive loss of immunoglobulins (nephrosis, severe burns, lymphangiectasia, severe diarrhea) |
|
Hyper IgM Immunodeficiency |
Carbamazepine |
Congenital infection with CMV |
Non Hodgkin's lymphoma |
|
|
Transcobalamin II deficiency and hypogammaglobulinemia |
Glucocorticoids |
Congenital infection with Toxoplasma gondii |
B cell malignancy |
|
|
X-linked agammaglobulinemia |
Fenclofenac |
Epstein-Barr Virus |
||
|
X-linked lymphoproliferative disorder (EBV associated) |
Gold salts |
|||
|
X-linked SCID |
Penicillamine |
|||
|
Some metabolic disorders |
Phenytoin |
|||
|
Chromosomal Anomalies |
Sulfasalazine |
|||
|
Chromosome 18q- Syndrome |
||||
|
Monosomy 22 |
||||
|
Trisomy 8 |
||||
|
Trisomy 21 |
https://esid.org/working-parties/registry-working-party/diagnosis-criteria/
Selective IgA deficiency
- Is common with a frequency of 1:500 of the general population (Swain et al., 2019).
- Thought to result from failure of differentiation of IgA producing B cells.
- Defined as undetectable IgA (usually <0.07 g/L) with normal other immunoglobulins in a patient aged over four years (Yel, 2010).
- Majority of patients (85-90%) are asymptomatic and identified incidentally.
- Further investigation is warranted in the small proportion of patients who have (Yazdani et al., 2017):
- Increased infections, especially recurrent sinopulmonary infections.
- GI tract disease including infections (Giardia) and coeliac disease.
- Autoimmune disease.
- Symptomatic IgA deficient patients may have other immune deficits (e.g. specific antibody deficiency) (Grosserichter-Wagener et al., 2020).
- Patients may be at higher risk of severe reactions to blood products due to presence of antibodies against IgA, although reaction rates are low and patients can usually be safely transfused (Vosughimotlagh et al., 2023).
- Treatment includes preventative measures to reduce infection and treatment of complications.
X-linked and autosomal recessive agammaglobulinaemia
- Most common form is X-linked due to mutation in btk protein normally required for B cells to mature in the bone marrow (Cardenas-Morales and Hernandez-Trujillo, 2022).
- Deficiency results in absent or low numbers of B cells in blood and lymphoid tissue:
- No tonsils or palpable lymph nodes.
- Results in severe deficiency of all immunoglobulin isotypes (agammaglobulinaemia).
- Presents in males with recurrent or severe bacterial infections of ears, sinuses and lungs, particularly with encapsulated organisms.
- Presents at about six months of age as maternal IgG antibodies acquired transplacentally are lost (Cooper et al., 2003).
- Diagnosis often delayed with average age of diagnosis of three to five years.
- Mainstay of treatment is immunoglobulin replacement therapy (IRT) and aggressive treatment of infections.
Hyper-IgM immunodeficiency syndrome
- Hyper IgM syndromes are a group of disorders which may be X-linked or autosomal recessive (Yazdani et al., 2019).
- Disorders cause defect of B cells in switching from making IgM to B cells making IgG, IgA and IgE.
- Most common cause (70%) due to deficiency of CD40 ligand (CD40L) which is X-linked (Inwald et al., 2000).
- Typically have normal to high serum levels of IgM but low other immunoglobulin classes.
- Often associated with neutropenia at presentation.
- In some cases, this may also be associated with susceptibility to opportunistic infections such as pneumocystis and cryptosporidium and patients should therefore only drink cooled, boiled water.
Treatment options include:
- Prophylactic immunoglobulin replacement therapy.
- Antibiotic prophylaxis, particularly against pneumocystis.
- Haematopoetic stem cell transplant.
Specific antibody deficiency (SAD)
- This is an incompletely defined disorder affecting patients with normal serum IgG levels who fail to produce a normal IgG antibody response following antigen challenge, particularly to polysaccharide antigens (most commonly unconjugated pneumococcal vaccine e.g. Pneumovax®) (Perez et al., 2017).
- These patients may require antibiotic prophylaxis.
- Immunoglobulin replacement therapy may be considered where there is evidence of recurrent or persistent infection or suppurative lung disease.
6. Complement disorders (including HAE)
Overview
Complement deficiencies represent 1% of PID in Australia and New Zealand (Kirkpatrick and Riminton, 2007).
- They are most commonly caused by C1, C2 and C4 deficiencies and C1 inhibitor deficiency (Sullivan, 2022).
- Clinical indications for investigation include recurrent bacterial infections, autoimmunity and recurrent angioedema.
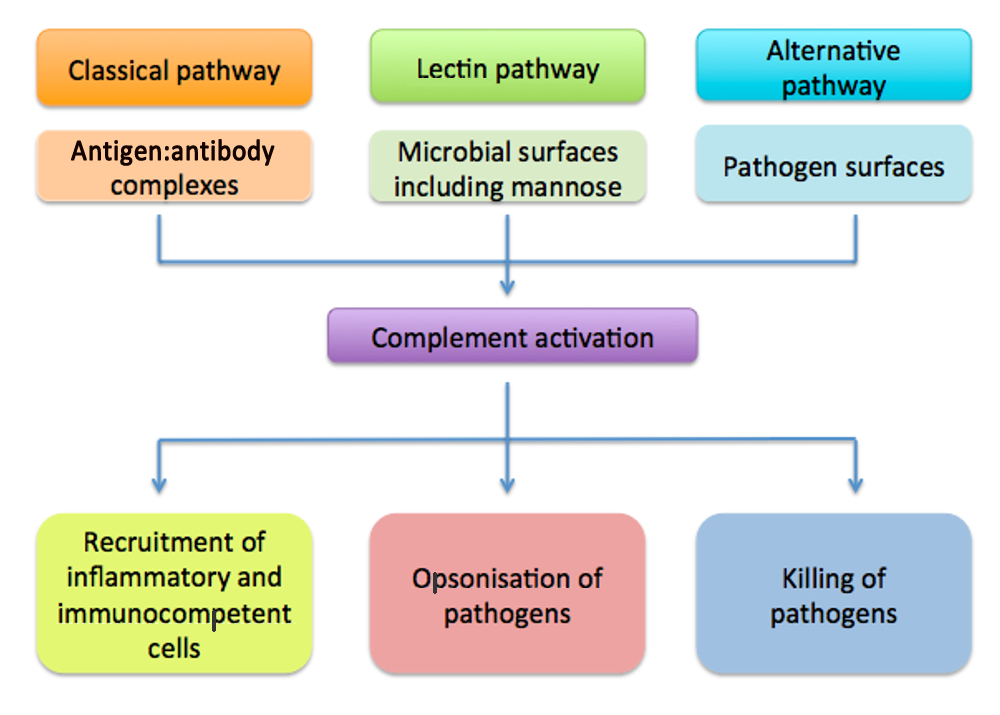
- Complement pathway consists of three main pathways: classical, alternative and mannose binding lectin pathway.
- All pathways converge on one common final or terminal pathway which generates membrane attack complex.
Infection associated/typical PID
There are a group of complement disorders that present with similar frequent infections as with other PID (Frank, 2010).
However, HAE does not present with the classic PID frequency of infection.
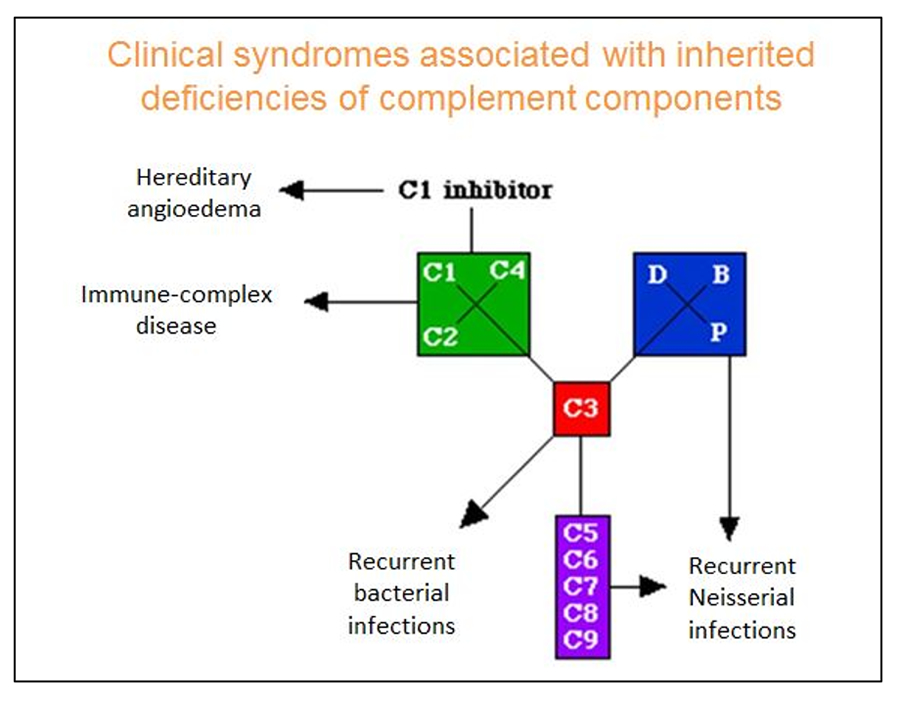
Deficiencies in classical pathway
- Classical pathway important in clearing of immune complexes and dead/dying cells (Truedsson, 2015).
- Primary deficiency of C1, C2 or C4 is linked to development of SLE.
- C2 deficiency most common complement deficiency in 1:20 000 (Sjöholm et al., 2006).
- Hereditary angioedema (HAE) due to C1 inhibitor deficiency, a control protein of classical pathway.
Deficiencies in alternative pathway
- Factor B and D deficiency are exceedingly rare (Kavanagh et al., 2025).
- Properidin deficiency is X linked and associated with increased susceptibility to Niesserial infections (Agarwal et al., 2010).
- Deficiencies of alternative pathway control proteins may cause consumption of complement and increase risks of infection and autoimmunity.
Deficiencies in mannose lectin pathway
- MBL binds to carbohydrates on microbes or unwanted material and triggers the activation of the lectin pathway (Takahashi et al., 2006).
- No consensus on the clinical relevance of MBL deficiency.
- The majority of individuals with low MBL levels are asymptomatic.
- However, increased susceptibility to infection with MBL deficiency appears to occur when there are other concomitant factors impairing the immune system.
Deficiencies in common final pathway
- Major feature in this group is recurrent infections (Xie et al., 2020).
- Generates membrane attack complex.
- Infection with Neisseria meningitides particularly common. Rare serotypes such a W135 and Y may cause disease.
Investigation of complement deficiency
- Clinical indications for investigation include recurrent bacterial infections and autoimmunity (Coss et al., 2023).
- HAE patients do not present with recurrent infections.
- Investigation of complement deficiency in consultation with clinical immunologist/specialist (McMurray et al., 2024):
- Initial screen for complement is C3, C4 levels and CH50 and may include AH50.
- CH50 assesses classical pathway and final common pathway.
- AH50 assesses alternative and final common pathway.
- Low CH50 alone suggests deficiency of C1, C2 or C4.
- Low AH50 alone suggests deficiency of Factor B, Factor D or Properidin.
- Low AH50 and CH50 suggests deficiency of C3 or final common pathway.
Hereditary Angioedema (HAE)
- Rare autosomal dominant genetic disorder due to deficiency of C1 inhibitor (C1 INH) protein (Campos et al., 2021).
- Patients suffer unpredictable and recurrent episodes of angioedema involving skin, gut and airway.
- Angioedema results from excessive production of bradykinin, a potent vasodilatory mediator (Germenis et al., 2020).
- Histamine and other mast cell mediators are not directly involved, which explains the lack of response to antihistamines.
- An acquired form of C1 INH deficiency may be seen in older patients without a family history of angioedema and is associated with underlying disorders and autoantibodies in most cases. Acquired C1-INH deficiency is not discussed further in this module (Trainotti et al., 2023).
Types of HAE
Type 1
- Type 1 HAE most common (85%) due to low levels of the C1 inhibitor (C1-INH) (Guan et al., 2024).
- Upon testing, protein (antigenic) and functional levels are both low.
Type 2
- Type 2 HAE (remaining 15%) due to normal levels but abnormal function of C1-INH (Abuzakouk et al., 2018).
- Upon testing normal or elevated protein levels but functional levels are low.
Type 3
- Distinguished from other types of HAE due to normal levels and function of C1 inhibitor (Miranda et al., 2013).
- Some cases associated with mutations of Factor XII
In both Type 1 and Type 2 cases, C4 levels are usually low due to consumption of complement.
Indications for screening
Indications for screening of C1 inhibitor disorders include the presence of one or more of the following (Gülbahar, 2021):
- Recurrent angioedema without urticaria.
- Unexplained recurrent episodes of self-limited, colicky, abdominal pain.
- A family history of angioedema.
- Unexplained laryngeal oedema (even a single episode).
- Low C4 levels, especially in the setting of angioedema.
Diagnosis
- C1 Inhibitor protein (antigenic) and functional levels should be assessed (Farkas et al., 2017).
- Abnormal results should be repeated to confirm.
- Low C4 level would be supportive.
- All diagnosed patients should be referred to a clinical immunologist with experience in managing HAE patients.
- Genetic testing is not required to confirm the diagnosis in adults.
- C1-INH levels are difficult to interpret in children younger than one year.
Testing family members
- Once a diagnosis of HAE has been made, testing of the patient’s children, parents, and siblings (if parents carry the mutation) should be encouraged (Fragnan et al., 2018).
- Although autosomal dominant approximately 25% of cases result from de novo mutations, so may not always have affected family members.
- Affected individuals may be asymptomatic, especially early in childhood.
HAE attacks
- Attack frequency is variable (Mendivil et al., 2023).
- Untreated patients may have attacks up to every one to two weeks.
- Swelling usually builds up over 24 hours, and may last three to four days or longer.
- Manifestations:
- Abdominal pain – nausea, vomiting, dehydration, diarrhoea or constipation.
- Swelling – face, limbs, genitals.
- Oropharangeal swelling – less frequent, but life threatening.
- No itching or urticaria.
- Does not respond to adrenaline, antihistamine or corticosteroids.
Prodromal symptoms
Occurs in up to 50% of HAE patients (Prematta et al., 2009; Magerl et al., 2018):
- Fatigue.
- Flu-like symptoms.
- Indigestion.
- Tingling or “tightness” sensation at site where swelling then occurs.
- About 25% of patients will have a flat, non-itching, blotchy, red rash before and during the attack.
Triggers of HAE include:
- Anxiety and stress
- Minor trauma or injury
- Some surgical and dental procedures
- Oestrogens
- ACE inhibitors
- Infections
- Sometimes no trigger factor can be identified
Zarnowski and Treudler (2024)
Treatment options for HAE
|
Drug |
Action |
Route |
|
Acute treatment |
||
|
Berinert Cinryze* |
C1-inhibitor concentrate |
IV |
|
Firazyr (icatibant)** |
B2 bradykinin receptor antagonist |
SC |
|
Prophylaxis |
||
|
Danazol |
Anabolic steroid |
Oral |
|
Cinryze* |
C1-inhibitor concentrate |
IV |
|
Tranexamic Acid |
Antifibrinolytic |
Oral |
*Not registered in NZ
** Not licensed for children below 12 years
Valerieva and Longhurst (2022)
Ongoing care
- Education about HAE is very important, particularly in newly diagnosed patients (Riedl et al., 2022).
- Trigger avoidance – triggers vary, but generally avoidance of trauma particularly to the face and upper respiratory tract and recognition and prompt treatment of oral and dental infections.
- Planning for acute treatment – all patients should be equipped with:
- Medical information jewellery.
- An ASCIA Management Plan for HAE allergy.org.au/health-professionals/papers/hereditary-angioedema
- Vaccinations – patients should be vaccinated against hepatitis B in case they require blood-derived products in the future (Craig et al., 2012).
Indications for prophylaxis
Short term prophylaxis should always be administered if intubation, oral surgery or general surgery is planned, as oral surgery is one of the most predictable triggers for HAE attacks (Farkas et al., 2012).
Long term prophylaxis is given to decrease the overall number of attacks (Li, 2020).
Further information
ASCIA HAE position statement and action plan
www.allergy.org.au/health-professionals/papers/hereditary-angioedema
HAE Australasia www.haeaustralasia.org.au/
7. Other PID syndromes
DiGeorge Syndrome
DiGeorge syndrome:
- Is due to defective embryonic development of the 3rd and 4th pharyngeal pouches, with the classic features of conotruncal abnormalities, thymic hypoplasia and hypoparathyroidism (Fomin et al., 2010).
- Is often associated with 22q11.2 deletion (which affects 1:4000 live births) but absence of 22q22.1 deletion does not exclude DiGeorge syndrome (De decker and Lawrenson, 2001) .
- May present with a wide phenotypic spectrum.
The spectrum of immune defect in patients with DiGeorge syndrome varies widely and is not related to other clinical features (McLean-Took et al., 2007):
- Most commonly mild to moderate decrease in T cells, and patients are not clinically immunodeficient.
- Antibody deficiencies can sometimes be seen, and may necessitate antibiotic prophylaxis or immunoglobulin replacement therapy.
- Autoimmunity can also be seen.
- Complete DiGeorge syndrome affects <1% of patients, who have complete absence of thymus. These patients have no T cell function and present with SCID phenotype, requiring HSCT or thymic transplant.
- Hypoplasia of parathyroid glands is associated with hypocalcaemia.
- Contruncal cardiac defects may occur including Tetralogy of Fallot, Ventricular septal defect and interrupted aortic arch.
- Patients almost always show abnormal facial features but may be subtle.
- 50% of patients will have ‘non typical’ malformations including opthalimic, renal, endocrine and musculoskeletal anomalies.
Diagnosis of DiGeorge syndrome involves karotyping and fluorescent in-situ hybridization (FISH) to look for 22q11.2 deletion (Larson and Butler, 1995).
Treatment of DiGeorge syndrome is dependent upon specific clinical features (Mustillo et al., 2023):
- Hypocalcaemia may require calcium supplements.
- Surgical correction of congenital heart defect usually needed.
- Immune reconstitution is not recommended for milder immunological defects.
- IVIg and antibiotic prophylaxis may be indicated dependent on immune function.
Wiskott-Aldrich Syndrome (WAS)
Wiskott-Aldrich (WAS) is an X-linked recessive disease due to mutation in the Wiskott-Aldrich Syndrome Protein (WASP) gene, which is important in cytoskeletal organisation of cells. Mutations can result in a wide spectrum of disease (Jin et al., 2004):
- Classic WAS was originally described as a triad of immunodeficiency, thrombocytopenia with small platelets and eczema.
- Severe disease results in a broad immunodeficiency (affecting both T and B cells, which may worsen with time), autoimmunity and malignancy.
- These patients may present with early opportunistic infection (e.g. PJP).
- Milder forms include isolated thrombocytopenia (XLT) or neutropenia (XLN).
Investigations typically show thrombocytopenia with small platelets, low serum IgM and poor pneumococcal antibody responses (Sudhakar et al., 2021).
Treatment may include (Buchbinder et al., 2014):
- Immunoglobulin replacement therapy and antibiotic prophylaxis may be indicated dependent on immune function.
- Bleeding problems may require platelet transfusions.
- Patients with severe disease require HSCT.
Transient hypogammaglobulinaemia of infancy (THI)
Development of immunoglobulins through infancy and early childhood follows a predictable pattern, with IgM reaching adult normal ranges in the first year of life, IgG by middle childhood, and IgA by adolescence (Justiz-Vaillant et al., 2023). However, in some infants and young children the production of immunoglobulins is delayed. These children may present with recurrent mild infections particularly in the first one to two years of life. The diagnosis of transient hypogammaglobulinaemia of infancy (THI) can only be securely made once immunoglobulin levels normalise, and these children need exclusion of more significant immunodeficiency.
Management for most children with THI involves periodic monitoring (e.g. six monthly) of immunoglobulin levels (Ameratunga et al., 2019). Prophylactic antibiotics may be needed, and rarely a defined period of immunoglobulin replacement may be considered, with a plan to reevaluate with time.
Chronic Granulomatous Disease (CGD)
Chronic Granulomatous Disease (CGD) is a rare disorder where neutrophils are able to ingest organisms but cannot kill them, resulting in granuloma formation (Roos, 2016). CGD is due to mutations in nicotinamide adenine dinucleotide phosphate (NADPH) oxidase complex which normally generates microbicidal respiratory bursts. CGD can present at any age, but most commonly during early childhood.
Patients with CGD are susceptible to recurrent and persistent infections with organisms which are resistant to non-oxidative killing. An X-linked mutation in gp91 component accounts for 65% of cases. Other causes are autosomal recessive, therefore girls can also be affected (Holland, 2013).
Patients with CGD usually present with (Staudacher and von Bernuth, 2024):
- Infections - frequent first manifestations including pneumonia, cellulitis and skin abscesses, liver abscesses and osteomyelitis (usually caused by Staphylococcus aureus).
- Fungal infections with Aspergillus may be seen as well as infections with unusual organisms such as mycobacteria and burkholderia.
- Development of granulomas in the skin, gastrointestinal (GI) tract, and genitourinary (GU) tract.
- Granulomatous inflammation of bowel which may cause granulomatous colitis, Crohns-like inflammatory bowel disease (IBD) or bowel obstruction.
Investigations include nitroblue tetrazolium (NBT) and/or Dihydrorhodamine (DHR) tests for neutrophil oxidase activity (Yu et al., 2018). Specialised protein or genetic tests are useful to establish subtype and genetic inheritance pattern.
Treatment options for CGD include (Oikonomopoulou et al., 2022):
- Antibacterial and antifungal prophylaxis.
- Gamma Interferon.
- Haematopoetic stem cell transplant (HSCT).
Hemophagocytic lymphohistiocytosis (HLH)
Hemophagocytic lymphohistiocytosis (HLH) is a life threatening syndrome of excessive immune activation (Kaçar and Celkan, 2022).
HLH (George, 2014):
- May occur as a primary (familial/hereditary) or secondary (non-familial/hereditary) disorder.
- Is characterised by hereditary or acquired impaired cytotoxic function of NK cells and cytotoxic T-cells.
- Impaired killing of target cells results in excess pro-inflammatory cytokine release (IFN-g, TNF-a, GM-CSF) from activated T cells and macrophages (cytokine storm).
- Presentation of primary HLH is often triggered by infections (especially EBV or CMV) or other metabolic/immune stress.
- Common genetic causes of primary HLH - Familial HLH (FHL) types 2-5 (perforin, MUNC13-4, syntaxin 11, syntaxin binding protein), X-linked lymphoproliferative syndrome (SAP (XLP1) or XIAP (XLP2)), Chediak-Higashi syndrome, Griscelli syndrome type 2, Hermansky-Pudlak syndrome type 2).
- Primary HLH usually develops during the first one to three years of life but late-onset cases can present at any age.
- Secondary HLH develops in association with infection, autoimmune / rheumatological conditions and malignancy.
- Molecular mechanisms involved in secondary HLH are unknown but may be predisposed to by polymorphisms in or carrier status of genes associated with primary HLH.
- Early diagnosis is important.
- Typical presentation with persistent fever, hepatic and/or renal dysfunction, pancytopenia, splenomegaly, hemorrhagic diathesis, neurological symptoms.
Diagnostic criteria for HLH
A. Molecular diagnosis consistent with HLH: pathologic mutations of PRF1, UNC13D, Munc18-2, Rab27a, STX11, SH2D1a, or BIRC4 (Chinnici et al., 2023)
or
B. Five of the eight criteria below are fulfilled (Henter et al., 2024):
- Fever ≥ 38.5°C
- Splenomegaly
- Cytopenias (affecting at least 2 of 3 lineages in the peripheral blood):
Hemoglobin <9 g/dL (in infants <4 weeks: hemoglobin <10 g/dL)
Platelets <100 x 103/ml Neutrophils <1 103/ml - Hypertriglyceridemia (fasting, >265 mg/dL) and/or hypofibrinogenemia (<150 mg/dL)
- Hemophagocytosis in bone marrow, spleen, lymph nodes or liver
- Low or absent NK-cell activity
- Ferritin >500 ng/mL
- Elevated sCD25 (α-chain of sIL-2 receptor)
HLH treatment
- Early aggressive supportive therapy and treatment of infection essential.
- Immunosuppressive therapy to control the hypercytokinemia with steroids, cyclosporin, intravenous immunoglobulin +/- etoposide or anti-thymocyte globulin (especially if primary HLH) - commonly used protocols are HLH-94 and HLH-2004 (Wu et al., 2024).
- Allogeneic hematopoietic stem-cell transplantation (HSCT) recommended for primary HLH or refractory secondary disease (Lehmberg et al., 2019).
- High morbidity and mortality (Marsh et al., 2010):
- Death during acute phase in 10%-15%,
- <10% 3 year survival if untreated,
- 21-26% 5 year survival with treatment,
- 43%-92% 3 year survival post HSCT.
© ASCIA 2025
ASCIA is the peak professional body of clinical immunology/allergy specialists in Australia and New Zealand.
ASCIA resources are based on published literature and expert review, however, they are not intended to replace medical advice. The content of ASCIA resources is not influenced by any commercial organisations.
For more information go to www.allergy.org.au
To donate to immunology/allergy research go to www.allergyimmunology.org.au
8. References
For Reference please download the PDF ![]() ASCIA HP Clinical Update PID 2025669.69 KB.
ASCIA HP Clinical Update PID 2025669.69 KB.
Content updated May 2025
