Adrenaline (Epinephrine) Injectors
Frequently Asked Questions
This document has been developed by ASCIA, the peak professional body of clinical immunology/allergy specialists in Australia and New Zealand. ASCIA information is based on published literature and expert review, is not influenced by commercial organisations and is not intended to replace medical advice. For patient or carer support contact Allergy & Anaphylaxis Australia or Allergy New Zealand.
![]() ASCIA PC Adrenaline Injector FAQ 2024388.93 KB
ASCIA PC Adrenaline Injector FAQ 2024388.93 KB
Q 1: Why is adrenaline needed to treat anaphylaxis?
Anaphylaxis is a medical emergency and adrenaline is a life saving treatment, which:
- Reverses a severe allergic reaction by treating signs and symptoms of anaphylaxis.
- Assists breathing, maintains heart function and blood pressure.
- Works within minutes and the effects last around 10 to 20 minutes.
- Is a hormone produced naturally in the body and is safe when given using an adrenaline injector.
Q 2: What are adrenaline injectors?
Adrenaline injectors are devices that:
- Contain a single fixed dose of adrenaline.
- Are designed to be used by people who do not have medical training.
- Are given by injecting into the outer mid-thigh (around halfway between the hip and knee).
- Can be given through a single layer of clothing (taking care to avoid seams, pockets and very thick clothing).
- Can be self-administered if the person having anaphylaxis has the ability and is not too unwell.
- Include instructions and an expiry date on the label.
Q 3: What adrenaline injectors are available in Australia and New Zealand?
ASCIA recommends the use of adrenaline (epinephrine) as the first line emergency/first aid treatment for severe allergic reactions (anaphylaxis) using either of the following two brands of adrenaline injectors:
- EpiPen® - Available in Australia (PBS* listed since 2003) and New Zealand (Pharmac** listed from 1 February 2023)
- Anapen® - Available in Australia (PBS listed since 2021).
*Australian Government Pharmaceutical Benefits Scheme (PBS)
The initial PBS authority prescription for up to two adrenaline injector devices can be provided to patients:
- Assessed to be at significant risk of anaphylaxis by a clinical immunology/allergy specialist, respiratory physician, or a paediatrician, or by a GP in consultation with one of these specialists; or
- After treatment with adrenaline for anaphylaxis when they are discharged from a hospital or emergency department.
A doctor (including a GP) or nurse practitioner can provide follow-up PBS authority prescriptions for replacement of devices just prior to expiry, or devices used for treatment of anaphylaxis.
** New Zealand Government Pharmaceutical Management Agency (Pharmac)
The initial Pharmac authority prescription for up to two adrenaline injector devices can be provided to patients:
- Assessed to be at significant risk of anaphylaxis by a relevant practitioner; or
- Who have experienced a previous anaphylactic reaction which has resulted in presentation to a hospital or emergency department.
Additional prescriptions are limited to replacement of devices just prior to expiry, or devices used for treatment of anaphylaxis.
Q 4: Why are two adrenaline injectors usually prescribed for people at risk of anaphylaxis?
- For children in CEC centres or school, having two adrenaline injectors means it is possible for one to be kept with the child at all times (whilst in or out of the home), and another to be kept at the CEC centre or school.
- For older children or adults weighing 50 kg or more, this allows two adrenaline injectors to be kept with them at all times, in case more than one dose of adrenaline is required.
- Ideally, people at risk of anaphylaxis should always have two devices available in case the ambulance is delayed during an emergency, and a second dose of adrenaline is required. As stated on the ASCIA Action Plan for Anaphylaxis: Further adrenaline may be given if there is no response after 5 minutes.
Q 5: Are adrenaline injectors available without a prescription?
Yes. Adrenaline injectors are available from pharmacies without a prescription at full retail price (not PBS subsidised).
If they are purchased directly from pharmacies without a prescription, you should request training from the pharmacist on how to use the adrenaline injector.
Q 6: Can an adrenaline injector be purchased for general use?
Yes. Many schools and some CEC centres, workplaces and restaurants purchase adrenaline injectors for general use which can be included in a first aid kit.
These general use devices should be:
- Given, if available, when a person having anaphylaxis does not have a prescribed device.
- Considered as an addition to prescribed adrenaline injectors, NOT a substitute for them.
- Stored with the ASCIA First Aid Plan for Anaphylaxis.
Follow the instructions on the device label or ASCIA First Aid Plan for Anaphylaxis.
Advice and training from the local education and/or health authorities should be sought regarding adrenaline injectors for general use.
Q 7: Why are multiple brands of adrenaline injectors required?
Both EpiPen® and Anapen® devices are widely used in other countries.
Most countries have multiple brands of adrenaline injector devices available, and this is important for the following reasons:
- To ensure continued supply of life saving adrenaline, particularly if one brand has stock shortages.
- To provide doctors with a choice of dose, who may prefer to prescribe a higher dose (500 microgram device) for people over 50kg.
- A 500 microgram device can potentially prevent the need for further doses of adrenaline (which is important due to increasing ambulance delays and many people carrying only one device).
- To encourage suppliers to provide devices with longer shelf life.
- To provide choice for consumers to access devices with points of difference to best suit their needs.
ASCIA Action Plans for Anaphylaxis that are specific for both EpiPen® and Anapen® are available.
Updated ASCIA Action Plans for Anaphylaxis, e-training courses and other resources which include EpiPen® and Anapen® instructions are available at www.allergy.org.au/anaphylaxis
Q 8: What doses of adrenaline injectors are prescribed in Australia and New Zealand?
ASCIA Guidelines* recommend the doses listed below, based on expert consensus and standard practice by ASCIA members, which are slightly different to the product information.
For children 7.5-20kg (aged around one to five years):
- EpiPen® Junior (150 microgram) or
- Anapen® 150 (Jr) (150 microgram)
For children over 20kg (aged around five years or over) and adults:
- EpiPen® (300 microgram) or
- Anapen® 300 (300 microgram)
For children over 50kg (aged around twelve years or over) and adults:
- Anapen® 500** (500 microgram) or
- Anapen® 300 (300 microgram) or
- EpiPen® (300 microgram)
*ASCIA Guidelines are consistent with the Acute Anaphylaxis Clinical Care Standard for Australia, Australian Prescriber Anaphylaxis wallchart, Australian Immunisation Handbook, and recommendations from the World Allergy Organisation (WAO), Canada and the UK.
**The dose of adrenaline in Anapen 500 is consistent with the intramuscular injection (IMI) dose recommendations for people who weigh more than 50kg in several publications, that are listed in the ASCIA Guidelines for Adrenaline Injector Prescription
Q 9: Can a different dose or brand be given if the prescribed adrenaline injector is not available?
Yes. As stated on the ASCIA Action Plan for Anaphylaxis, if in doubt give adrenaline injector (Anapen® or EpiPen®) using the following dosing guide:
- For children 7.5-20kg (around one to five years of age), a device containing 150 micrograms of adrenaline should be used. However, if only a higher dose device is available (containing 300 micrograms of adrenaline) this should be used in preference to not using one at all. Under-treatment of anaphylaxis is more harmful (and potentially life threatening) than over-treatment of a mild or moderate allergic reaction. If the only device available in an anaphylaxis emergency is an Anapen®500 and the child is 20 kg or under, contact emergency services. Ask a paramedic or medical officer to provide advice/guidance prior to proceeding with treatment before their arrival.
- For children over 20kg (aged around five years or over) and adults, a device containing 300 micrograms of adrenaline should be used. However, a 500 microgram device can be given if an injector containing 300 micrograms of adrenaline is not available.
- For children (aged around twelve years or older) and adults over 50kg, a device containing either 300 micrograms (Anapen® or EpiPen®) OR a device containing 500 micrograms (Anapen) should be used. However, if only a lower dose device is available, this should be used in preference to not using one at all.
- For children or adults over 50kg an injector containing 500 micrograms of adrenaline may be prescribed. However, if an injector containing 500 micrograms of adrenaline is not available, then a 300 microgram device can be given to any child or adult over 20kg. Call emergency services regarding management if no adrenaline injector is available or if an adrenaline injector has been administered.
As stated on the ASCIA Action Plans, further adrenaline may be given if no response after 5 minutes:
- If 300 micrograms of adrenaline have been given as a first dose and the child (over 20kg) or adult requires a second dose of adrenaline, the adrenaline device that is available should be given.
- If 150 micrograms of adrenaline have been given as a first dose and the child (under 20kg) requires a second dose of adrenaline (if symptoms of anaphylaxis persist or recur), a 300 microgram dose of adrenaline may be given if a 150 microgram adrenaline injector isn’t available.
- Call emergency services if no adrenaline injector is available or if an adrenaline injector has been given.
Q 10: Why are adrenaline injectors recommended in non-medical settings?
ASCIA recommends that adrenaline injectors are used to treat anaphylaxis in schools, children’s education/care (CEC) centres, and any other non-medical settings, to avoid delay in adrenaline administration and ensure that the correct dose of adrenaline is given.
Students and children may be prescribed either an EpiPen® or Anapen® device so both must be accepted, with school and CEC services staff trained to administer either brand.
Adrenaline ampoules, needles and syringes are used by trained health professionals to treat anaphylaxis. However, this is an advanced skill that is only appropriate in a medical setting. In non-medical settings such as schools, CEC centres, and workplaces, adrenaline injectors are the safest and most appropriate device to use when administering first aid.
Q 11: How do you give an EpiPen®?
There are three steps to give EpiPen® as shown below:

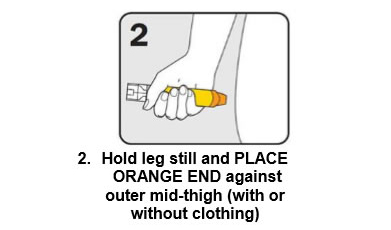
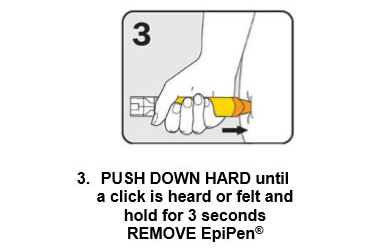
Give the ambulance staff the used EpiPen® and tell them the time it was given.
EpiPen® is available in Australia (PBS listed) and New Zealand (Pharmac listed).
Q 12: How do you give an Anapen®?
There are four steps to give Anapen® as shown below:
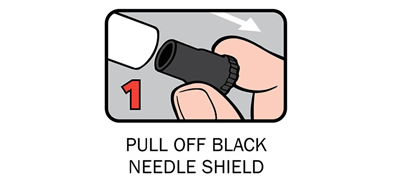
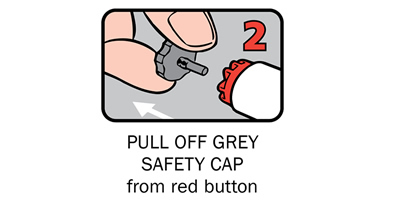
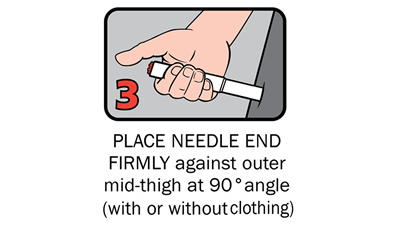
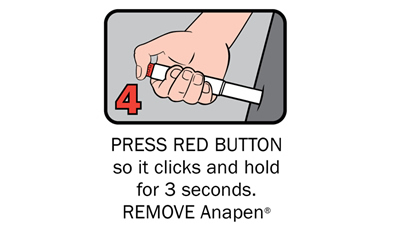
After phoning an ambulance, place the used Anapen® in a hard plastic container (where possible) or place needle into wide end of the black needle shield. Give the ambulance staff the used Anapen® and tell them the time it was given.
Anapen® is available in Australia (PBS listed).
Q 13: Who can give an adrenaline injector?
Adrenaline injectors can be used by people without medical training in an anaphylaxis emergency. This includes a friend, teacher, children’s education/care (CEC) centre worker, parent, other relative or the person with anaphylaxis themselves (if they are well and old enough).
Instructions are shown on the label of each device and on the ASCIA Action Plan for Anaphylaxis.
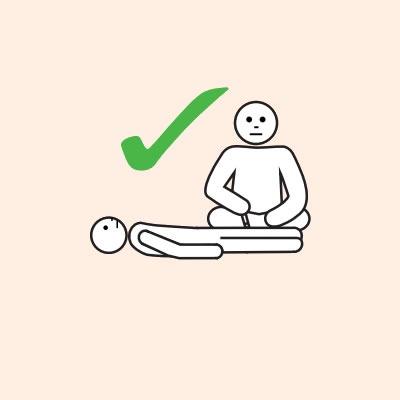
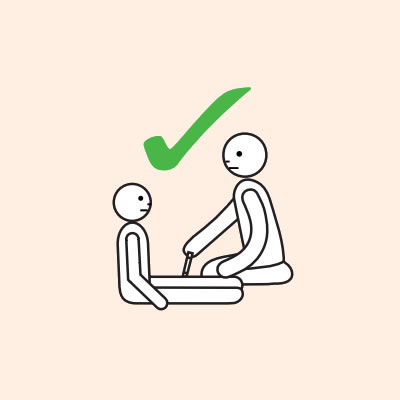
- To give the adrenaline injector you may need to kneel beside the person and hold their leg still, then give the adrenaline injector.
- If another person is present, they can assist by phoning an ambulance and/or assisting with CPR if required.
Some older children or adults can self-administer adrenaline injectors if they are not too unwell or unconscious:
- They should lay flat once it has been given, or sit with legs outstretched if breathing is difficult, or in the recovery position if they are vomiting or pregnant.
- If a person who knows how to self-administer is too unwell, they will need another person to give the adrenaline injector.
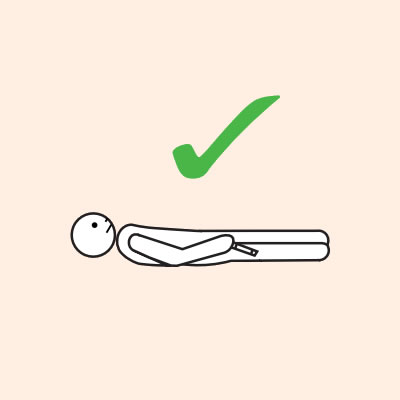
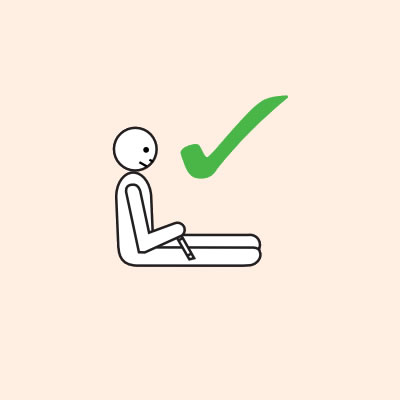
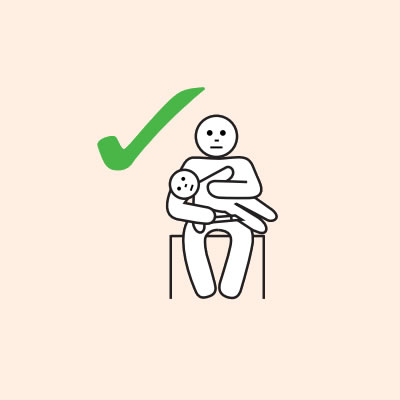
Q 14: If you are unsure if it is anaphylaxis, should you use an adrenaline injector?
Yes. If in doubt, it is better to use an adrenaline injector than not use it:
- Under-treatment of anaphylaxis is more harmful (and potentially life threatening) than over-treatment of a mild or moderate allergic reaction.
- Further adrenaline may be given if there is no response after 5 minutes.
Temporary side effects of adrenaline can include increased heart rate, trembling and paleness. Someone may still look unwell even after the adrenaline injector has been given.
Q 15: I am unsure whether it is asthma or anaphylaxis. Can an adrenaline injector still be used?
Yes. For people with asthma, who are also at risk of anaphylaxis, the adrenaline injector should always be given first followed by the asthma reliever puffer and calling an ambulance. Asthma first aid should continue, and it is important to keep following the ASCIA Action Plan for Anaphylaxis.
If someone with known food or insect allergy suddenly develops severe asthma-like symptoms, always give the adrenaline injector first, then asthma reliever puffer.
Q 16: Can adrenaline be given if CPR has commenced?
Yes. Adrenaline should always be given before CPR commences. However, it may not always be immediately available. If CPR has started, and adrenaline has not yet been administered, give it as soon as it is available.
There is no need to stop CPR while the adrenaline injector is being given if there is a second person there to assist. If no adrenaline injector is available, CPR must continue until emergency services arrive.
Q 17: Why do adrenaline injectors need to be administered into the outer mid-thigh?
Injecting adrenaline into the muscle of the outer mid-thigh ensures:
- The adrenaline is delivered into the muscle tissue where it will be rapidly absorbed, allowing it to work quickly.
- It very unlikely that damage to nerves and tendons will occur, or that it will be accidentally injected into an artery or vein.
This is shown in the diagrams on the adrenaline injector labels and the ASCIA Action Plans for Anaphylaxis.
Adrenaline injectors can be given through a single layer of clothing, but not through seams, pockets, or very thick clothing.
Another adrenaline injector (if available) may be given if there is no response after 5 minutes. There is no evidence that injecting the second dose of adrenaline into the same thigh is a problem. The most important thing is to give another dose of adrenaline (after 5 minutes) if symptoms persist, and phone an ambulance.
Q 18: What needs to be done after using an adrenaline injector?
- Phone ambulance - 000 (AU) or 111 (NZ).
- Phone family/emergency contact.
- Further adrenaline may be given if no response after 5 minutes.
- Commence CPR at any time if the person is unresponsive and not breathing normally.
- Transfer person to hospital (or other medical facility) for at least 4 hours of observation.
- Give ambulance staff the used device and the time it was given.
- The person having anaphylaxis should not be allowed to stand, sit up suddenly, or walk, even if they look like they have recovered. They should be carried on a stretcher or trolley bed to the ambulance.
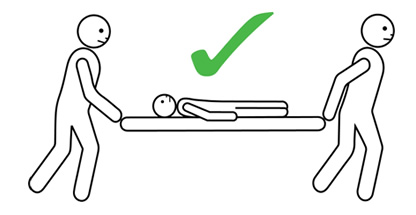
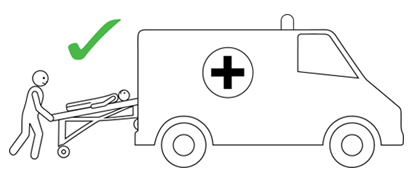
Q 19: How should a person with anaphylaxis be positioned?
Before, during and after using adrenaline, it is important to lay a person flat.
- When a person has anaphylaxis their blood pressure can drop rapidly, which reduces blood flow to the heart.
- Laying the person flat will help blood flow to the heart which improves blood pressure.
- Standing can make anaphylaxis worse by causing blood pressure to drop.
It is important to LAY PERSON FLAT - do NOT allow them to stand or walk.
If unconscious or pregnant, place in recovery position - on left side if pregnant.
- If breathing is difficult allow them to sit with legs outstretched.
- Hold young children flat, not upright.
- The person should NOT stand, walk, or be held upright, even if they appear to have recovered.
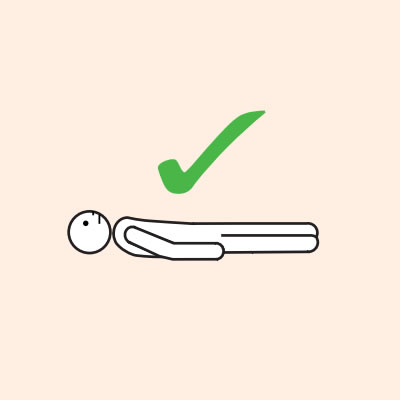
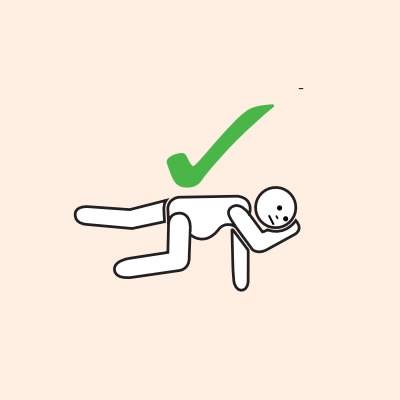
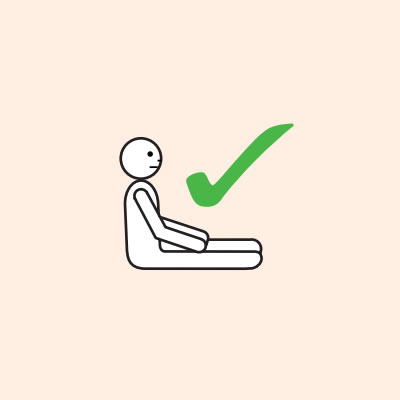
Do not allow the person experiencing anaphylaxis to walk to their medication, instead, the adrenaline injector should be brought to them as quickly as possible.
Q 20: What should people avoid doing when they have anaphylaxis?
Do not allow the person to take a shower. Under no circumstances should a person with anaphylaxis take a shower, even if they feel very hot, for the following reasons:
- Standing can cause a further drop in blood pressure.
- Warm showers promote vasodilation (widening of the blood vessels), which can also lower blood pressure.
- Bathroom floors are hard, so there is a greater risk of injury if the person faints and falls.
Do not allow the person to eat or drink anything. This can cause them to vomit, which may be inhaled (aspirated).
Q 21: Can the adrenaline injector device be reused?
No. Each adrenaline injector releases a single, fixed dose of adrenaline once the device is triggered. The adrenaline is expelled quickly once the device is activated, which can only be done once.
Q 22: What precautions should be taken when using an adrenaline injector?
It is important to follow the instructions, ensure that the needle end of the adrenaline injector is on the person's outer mid-thigh and that the needle is not touched after administration, to avoid needle stick injury. There are no situations where adrenaline should not be given to treat the person who is having anaphylaxis.
Q 23: Where should adrenaline injectors be stored?
Adrenaline injector devices should be stored:
- Where they are readily available and not in a locked cupboard.
- In a cool dark place at room temperature, between 15-25°C, but not refrigerated, as temperatures below 15°C may damage the injector mechanism.
- In an insulated wallet if a person carrying a device is outside for an extended time in the heat (such as hiking or at the beach).
- With a RED ASCIA Action Plan for Anaphylaxis, clearly labelled with the name of the person who has been prescribed the device, including if the device is carried by the person.
- With an ORANGE ASCIA First Aid Plan for Anaphylaxis if they are for general use (not prescribed for a person).
Q 24: At what age can students carry their own adrenaline injector at school?
The decision as to whether a student can carry their own adrenaline injector should be made when developing the student’s anaphylaxis management plan, in consultation with the student, their parents/guardians and their medical practitioner. This decision is generally based on a combination of factors, including age, maturity, and ability to use the device.
If a student carries their own adrenaline injector device, they:
- Should be educated that if they self-administer, they should immediately alert a staff member and an ambulance must be called.
- Need to have a second adrenaline injector (provided by the parent/guardian) kept on site at the school in an easily accessible, unlocked location that is known to all staff.
- May not physically be able to self-administer due to the effects of anaphylaxis.
Q 25: When do adrenaline injectors expire?
The shelf life of adrenaline injectors is normally one to two years from date of manufacture. The expiry date on the side of the device needs to be marked on a calendar and the device must be replaced prior to this date. Registration with a reminder service may assist, to provide reminders about expiry dates.
Q 26: Can an expired, cloudy, or discoloured adrenaline injector be used?
Adrenaline injectors with discoloured adrenaline or expired adrenaline injectors are not as effective and should not be relied on to treat anaphylaxis. However, the most recently expired adrenaline injector available should be used if there is no in-date device available.
Q 27: What documents are needed to take an adrenaline injector in airline flight hand luggage?
ASCIA has developed a Travel Plan for people at risk of anaphylaxis which needs to be completed and signed by the person's treating doctor and attached to the ASCIA Action Plan for Anaphylaxis.
The patient support organisation Allergy & Anaphylaxis Australia, also has information on travelling with severe food allergies: allergyfacts.org.au
People should carry their adrenaline injector in a container which includes a pharmacy label, particularly if travelling in the USA.
They should notify their travel agent, insurer and airline of their allergy and the need to carry an adrenaline injector in case additional documentation or preparation is required.
Further information about travelling with allergies, including a checklist, is available from the ASCIA website.
Q 28: Why are adrenaline injector training devices used?
It is important to have regular practice using both brands of adrenaline injector trainer devices* which:
- Do not contain adrenaline and do not have a needle, so they do not expire and can be reused.
- Should be stored in a different location than the real devices and clearly labelled as trainer devices.
- Can be purchased from the device supplier, pharmacies or patient organisations.
- Should be used for regular** practice to ensure correct use in an emergency.
* EpiPen® trainer devices have a grey label, Anapen® trainer devices have a white label
** Best practice guidelines recommend at least twice each year - www.allergy.org.au/schools-childcare
Q 29: Where can online training be accessed?
ASCIA Anaphylaxis e-training including short refresher courses can assist with training and are available free of charge at www.allergy.org.au/patients/anaphylaxis-e-training-schools-and-childcare
Q 30: Where can I find more information?
Adrenaline injector suppliers for Australia and New Zealand:
- Viatris - EpiPen® myepipen.com.au
- Arrotex - Anapen® anapen.com.au
© ASCIA 2024
Content updated March 2024
For more information go to www.allergy.org.au/patients/about-allergy
To support allergy and immunology research go to www.allergyimmunology.org.au/donate
